At its core, nutrition is so very basic: eat whole foods and avoid processed garbage. But when you look at how nutrition can improve performance, things get more complicated.
Human performance can be enhanced from a variety of ways. The most important piece is nutrition, not only because we eat multiple times per day, but also because as humans we want to live a long and healthy life. One of the best ways we can do this is by eating to become more alkaline.
Here we will explore what being acidic versus alkaline means, why you should care, and how you can eat to become more alkaline to improve performance and long-term health.
Fresh lemon juice is extremely acidic and can help keep your body’s pH closer to neutral.
The pH Scale
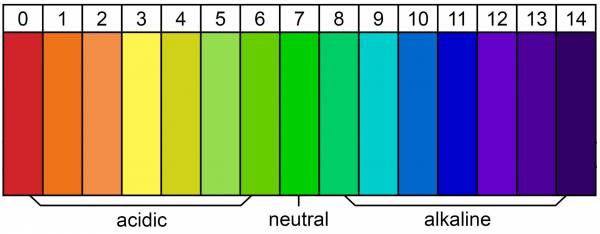
First, let’s discuss the pH scale. The pH scale tells us how many hydrogen ions (H+) or hydroxyl ions (OH-) are in a substance. The greater number of H+ ions, the greater the acidity. The greater the number of OH- ions means the substance is more alkaline.
An ion is unstable. It either has too many or too few electrons. If it has a positive charge like H+, it will steal electrons. If it has a negative charged OH-, it will want to give one away. This is why both acids and bases can cause chemical reactions.
The lower the number on the pH scale, the higher the acidity. The higher the number, the more alkaline it is. As you can see in the chart below, a pH of 7 is neutral.
Ideal pH in the Body
Your blood operates in a very narrow pH range, between 7.35 and 7.45 (slightly alkaline). If you go too far below or above this pH range, death will eventually be the result.1 The human body does an amazing job at buffering pH and preventing your blood from going outside of this range because it wants to continuously be in that optimal alkaline state. Your muscles, like your blood, prefer to be in a slightly alkaline state. However, unlike blood, the pH of muscle is more variable.2,3,4 This variability can have an effect on your physical performance.
Everything you eat influences your pH. However, the pH of your food doesn’t always correlate with the effect it has on the body once it is metabolized. Lemon, for example, is extremely acidic (pH of 2), but it has a powerful alkaline effect in the body. Foods that contain large amounts of nitrogen, chlorine, and phosphorus (e.g, meat) tend to be acid forming. Foods rich in calcium, potassium, sodium, and magnesium (e.g., leafy greens) tend to be alkaline forming.5
How Does pH Impact Performance?
Since your tissues have a more variable pH range, they can become acidic. This can happen due to the blood becoming more acidic (acidic diet, stress, improper breathing) and expelling excess acid into the connective tissue so the blood maintains a normal pH range and you don’t die. Acidity can also be caused by muscle tightness and knots (contracted sarcomeres), which do not allow proper blood flood and excretion of metabolic waste.6
Think about a hard workout and the feeling of your muscles burning. That muscle burn is caused by the production of anaerobic energy (without oxygen). This reaction causes the pH of your tissues to decrease, increasing acidity.2 This increasing acidity causes temporary muscle fatigue, limiting contractile force.7 Therefore, if your muscles have a higher resting pH, it takes longer for you to feel the burn, which means you are able to work harder and longer for more potential output.
When your blood pH drops and your body becomes too acidic, your body will take calcium from your bones to buffer that acidity to become more alkaline. 8 Chronic acidity can lead to deficiencies in essential minerals like magnesium, calcium, and potassium9 that can lead to many short- and long-term health challenges. Maintaining adequate levels of minerals supports good health, proper nerve function, and adequate recovery from exercise.
Raising your pH increases mineral stores, including magnesium (Mg) levels,10 which is critical for performance because magnesium is needed for 300 different enzymatic reactions, including proper energy production and utilization. ATP cannot be used unless it is bound to magnesium, so magnesium is essential if you want enough energy to hit your training hard. Further, raising your pH increases adiponectin levels and decreases cortisol.10
Eat to Become Alkaline
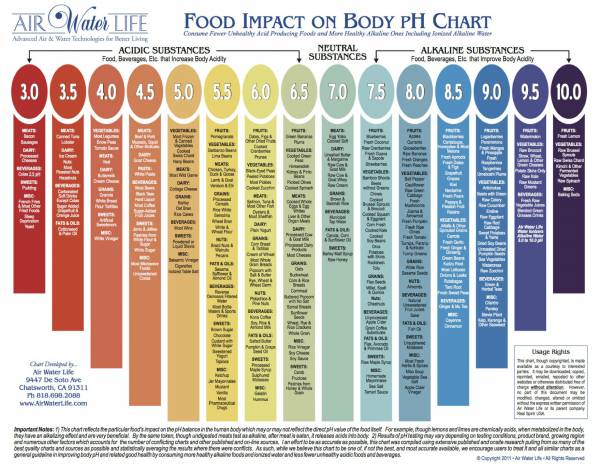
Foods leave either an acidic or alkaline residue in the body after you eat them. A good food intake guide for athletes is 80 percent alkaline, 20 percent acidic. Check out the chart below for a guide of what foods leave what kind of residue.
Click on the image for a closer look.
Foods to Avoid: Limit white sugar, alcohol, and factory-farmed animal products. These foods are extremely acidic and must be buffered by your stored minerals. An occasional beer can be part of your 20 percent.
Morning Routine: Drink a tall glass of warm water with the juice from half of a lemon. This is a good way to raise your pH first thing in the morning, as your normal metabolic processes have been dropping your pH as you sleep. Lemon also has antiseptic and antibacterial properties that will help cleanse your digestive tract and stimulate the flow of gastric juices and liver enzymes. Rinse your mouth out with water after you drink it, as the lemon juice can eat away at your enamel.
Deep Breathing: Carbon dioxide is acidic, so you do not want large amounts of it in your body. Practice deep breathing to help expel excess CO2 and oxygenate your body.
Balance Your Nutrition
Eating for optimal acid and alkaline balance is a simple way you can improve your performance today and increase your ability to fight disease and illness in the long term. By knowing what foods have either an acidic or alkaline effect, you can eat in a way that encourages a proper balance.
You’ll Also Enjoy:
- Diaphragmatic Breathing Reduces Exercise Induced Oxidative Stress
- Understanding Energy Systems: ATP-C, Glycolytic, and Oxidative
- Supplement Face-Off: Baking Soda Versus Beta-Alanine
- New on Breaking Muscle Today
References:
1. Annemarie Colbin, Food and Healing. (New York:Ballantine Books, 1986).
2. Lisa Hermanen and Jan-Bjorn Osnes, “Blood and muscle pH after maximal exercise in man,” Journal of Applied Physiology, 32(1972):304-308.
3. K. Sahlin, R.C. Harris, B. Nylind, E. Hultman, “Lactate content and pH in muscle samples obtained after dynamic exercise,” European Journal of Physiology, 28(1976):143-149.
4. Kent Sahlin, Roger Harris, Eric Hultman, “Creatine kinase equilibrium and lactate content compared with muscle pH in tissue samples obtained after isometric exercise,” Biochemical Journal, 152(1975):173-180.
5. Patrick Holford, The Optimum Nutrition Bible (Berkeley, CA: Crossing Press, 2004).
6. Clair Davies, Amber Davies, The Trigger Point Therapy Workbook, 2013.
7. Shannon T Knuth, “Skeletal muscle fatigue: pH effects on contractile function and excitation-contraction coupling in single cells” (January 1, 2000). Dissertations (1962 – 2010) Access via Proquest Digital Dissertations. Paper AAI9977733.
8. DJ Chambers, Hunman, J, et al, “The effect of ionized calcium, pH and temperature on bioactive parathyroid hormone during and after open-heard operations,” Annals of Thoracic Surgery, 36(1983): 306-313.
9. Gerry K. Schwalkenberg, “The Alkaline Diet: Is there Evidence That an Alkaline pH Diet Benefits Health?” Journal of Environmental Public Health (2012):727630. doi: 10.1155/2012/727630.
10. Ian Forrest Robey, “Examining the relationship between diet-induced acidosis and cancer,” Nutrition & Metabolism, 9(2012):72.
Photo 1 courtesy of Wikimedia Commons.
pH chart courtesy of Breaking Muscle.
Food impact chart courtesy of Air Water Life.